Acid-Base Balance Physiology-Review & Illustrations
General scheme for regulation of acid-base balance
Two types of acid are produced in the body: volatile acid
and nonvolatile acids.
1. Volatile acid
- is CO2.Is produced from the aerobic metabolism of cells.
- CO2 combines with H2O to form the weak acid H2CO3, which dissociates into H+ and HCO3– by the following reactions: CO2 + H2O ↔ H2CO3 ↔ H+ + HCO3-
- Carbonic anhydrase, which is present in most cells, catalyzes the reversible reaction between CO2 and H2O.
2. Nonvolatile acids
- Are also called fixed acids.
- Include sulfuric acid (a product of protein catabolism) and phosphoric acid (a product of phospholipid catabolism).
- Are normally produced at a rate of 40–60 mmoles/day.
- Other fixed acids that may be overproduced in disease or may be ingested include ketoacids, lactic acid, and salicylic acid.
Titration curve of bicarbonate buffer system:
If we plot a titration curve for the
bicarbonate buffer system (see figure) in which the
x-axis represents the pH of the solution and the y-axis represents the
percentage of added HCO3- (as a base) or CO2 (as
an acid) to the solution we will see that at the central point of the curve
(pK) the addition of a slight amount of acid or base causes minimal
change in pH. However toward each end of the curve addition of a slight amount
of acid or base causes the pH to change greatly.
Thus buffering power of the buffer system is greatest when:
- The pH of the buffer system operate is equal to the pK of the buffer, which is in the exact center of the curve (i.e., in the linear portion of the titration curve). Therefore for equal concentrations of the HCO3- and CO2 the pH of the solution is equal to the pK (6.1) which represents the pH at which the buffer system can operate with its maximum buffering power.
- The concentrations of the two elements of the buffer solution, the HCO3-and CO2. Thus the buffering power of a buffer is also directly proportional to the concentrations of the buffer substances.
Defense against change in [H+]:
To prevent acidosis or alkalosis,
several control systems are available:
1. Acid-base buffer system: Which are present in all body fluids that
immediately combine with any acid or alkali and thereby prevent excessive
change in [H+]. This system can act within a fraction of a second
to prevent excessive changes in [H+].
2. Respiratory center: Upon the changes in [H+] the
respiratory center is immediately stimulated to alter the rate of breathing. As
a result, the rate of CO2 removal from the body fluids is automatically changed
and this causes the [H+] to return toward normal. This mechanism
takes 1 to 15 minutes to readjust the [H+] after sudden
changes have occurred .
3. Kidneys: when the [H+] changes from normal the kidneys
excrete either an acid or alkaline urine thereby also helping readjust the [H+]
of the body fluids back to normal. The kidneys provide the most powerful of all
the acid-base regulatory systems but requires many minutes to several days
to readjust the [H +].
Acid-base buffer system of body fluids (see figure):
The three major buffer systems of the body fluids are the
bicarbonate buffer, the phosphate buffer, and the protein buffer. For the
blood, the buffering powers of various buffers are as follow:
- The bicarbonate buffer system → 53% (plasma 35% and RBC 18%)
- Phosphate
buffer system → 2%
- The protein buffer system → 45% (Hb 35% and plasma proteins 10%)
[1] The bicarbonate buffer system:
- It consist of mixture of carbonic acid (H2CO3) and sodium bicarbonate (NaHCO3) (K- or Mg- bicarbonate intracellularly).
- The bicarbonate buffer system is not powerful buffer for two reasons: (a) The pH in the extracellular fluids is about 7.4, while the maximum buffering power of this system is around pH 6.1 (pK 6.1). (b) The concentrations of the two elements of the bicarbonate system, CO2 and HCO3- are not great.
- Despite the fact that the bicarbonate buffer system is not especially powerful, it is actually more important than all the others in the body. This is because the concentration of each of the two elements of the bicarbonate system can be regulated, carbon dioxide by the respiratory system and bicarbonate ion by the kidneys.
[2] Phosphate buffer system:
- It is composed of NaH2PO4 and Na2HPO4.
- The maximum buffering power of this system is around pH 6.8 (pk = 6.8) which is not far from the normal pH of 7.4 in the body fluids, this allows the phosphate buffer system to operate near its maximum buffering power.
- its concentration in the extracellular fluid is only 1/12 those of the bicarbonate buffer. Therefore, its total buffering power in the extracellular fluid is even far less than that of the bicarbonate system.
- The phosphate buffer is especially important in:
[1] The tubular fluid of the kidneys for two reasons: (a) Phosphate usually becomes greatly concentrated in the tubules (because of their relative poor reabsorption and because of removal of water from the tubular fluid) thereby also greatly increasing the buffering power of the phosphate system. (b) The tubular fluid usually becomes more acidic than the extracellular fluid, bringing the operating range of the buffer closer to the pH for the maximum buffering power of this system.
[2] The intracellular fluids because the concentration of phosphate in these fluids is many times that in the extracellular fluids, and also because the pH at which this system operate at its maximum power is closer to the pH of intracellular fluid than the pH of the extracellular fluid.
[3] The protein buffer system:
- Proteins and the amino acids of which they are formed, possess both acidic groups, usually the -COOH radical, and basic groups, usually the -NH2 radical.
- It is the most plentiful buffer of the body due to the high concentrations of the protein of the cells and plasma.
- Hb is a particular good buffer. The remarkable buffering capacity of Hb is due to the fact that Hb molecule has 38 units of Histidine (amino acid) which contains additional -NH2 and -COOH groups. As a result, Hb is a particular good buffer.Hb in the oxy-forms (oxy-Hb-) is a stronger acid than in the reduced (deoxy) form (Hb-).
Respiratory regulation of acid-base balance
Because of the ability of the
respiratory center to respond to blood CO2 concentration and H+
concentration, and because changes in alveolar ventilation in turn alter
the blood CO2 concentration and H+ concentration in the
fluids, the respiratory system acts as a typical feedback regulatory system for
controlling H+ concentration.
Renal regulation of acid-base
balance:
The kidneys regulate H+ concentration principally by increasing or decreasing the HCO3- concentration in the body fluid. This is achieved by the following mechanisms:
1. Reabsorption of filtered HCO3– (Figure): It occurs primarily in the proximal tubule. Key features of reabsorption of filtered HCO3–:
- (A) H+ and HCO3− are produced in the proximal tubule cells from CO2 and H2O. CO2 and H2O combine to form H2CO3, catalyzed by intracellular carbonic anhydrase; H2CO3 dissociates into H+ and HCO3–. H+ is secreted into the lumen via the Na+-H+ exchange mechanism in the luminal membrane. The HCO3– is reabsorbed.
- (B) In the lumen, the secreted H+ combines with filtered HCO3– to form H2CO3, which dissociates into CO2 and H2O, catalyzed by brush border carbonic anhydrase. CO2 and H2O diffuse into the cell to start the cycle again.
- (C) The process results in net reabsorption of filtered HCO3–. However, it does not result in net secretion of H+.
Increases in the filtered load of HCO3– result in increased rates of HCO3– reabsorption. However, if the plasma HCO3– concentration becomes very high (e.g., metabolic alkalosis), the filtered load will exceed the reabsorptive capacity, and HCO3– will be excreted in the urine.
- Increases in PCO2 result in increased rates of HCO3– reabsorption because the supply of intracellular H+ for secretion is increased. This mechanism is the basis for the renal compensation for respiratory acidosis.
- Decreases in PCO2 result in decreased rates of HCO3– reabsorption because the supply of intracellular H+ for secretion is decreased. This mechanism is the basis for the renal compensation for respiratory alkalosis.
- ECF volume expansion results in decreased HCO3− reabsorption.
- ECF volume contraction results in increased HCO3− reabsorption.
- Angiotensin II stimulates Na+–H+ exchange and thus increases HCO3− reabsorption, contributing to the alkalosis that occurs secondary to ECF volume contraction.
[2] Incomplete titration of secreted H+ against filtered HCO3- (see figure) : Under normal conditions, the rate of H+ secretion is about the rate of filtration of HCO3- in the glomerular filtrate, thus, the quantities of the two ions entering the tubules are almost equal, and they combine with each other and actually titrate each other in the tubules, the end – products being CO2 and water (see the figure above). Thus, the basic mechanism by which the kidney corrects either acidosis or alkalosis is by incomplete titration of H+ against HCO3-, leaving one or the other of these to pass into the urine and therefore to be removed from the extracellular fluid. The majority of the excess urinary H+ is usually carried by combination with "intratubular buffers" which transport the excess H+into the urine and these are:
- The phosphate buffer,
- Ammonia buffer system
This ammonia buffer system is
especially important for two reasons:
- By this buffer system the rate of NH3 secretion into the tubular fluid is actually controlled by the amount of excess H+ to be transported.
- When H+ combine with NH3 and the resulting NH4+ then combine with chloride the pH does not fall significantly because NH4CI is only very weak acidic. If the tubular pH falls rapidly below the critical value of 4.5, further H+ secretion is inhibited.

Normal values:
- pH = 7.35 - 7.45
- Blood PCO2 = 35-45 mmHg
- Plasma HCO3 ̶ = 22-28 mEq/L
- [HCO3–]/[CO2] ratio = 20:1
1. Respiratory acidosis (CO2 retention):
Etiology:
- Any factor that interferes with the gaseous exchange between the blood and the alveolar air can cause respiratory acidosis such as airway obstruction, pneumonia, etc.
- Any factor that causes a reduced breathing that may result from depression or damage to the respiratory center or skeletal deformities or paralysis of respiratory muscles that causes reduced breathing. Respiratory acidosis may be achieved temporarily by voluntary breath holding.
2. Respiratory alkalosis (CO2 washout or deficit):
Etiology:
- Hysteria.
- Over-ventilation in a mechanical respirator or Injury of respiratory center in the course of meningitis or encephalitis.
- In early stage of salicylate (aspirin) intoxication due to pharmacological stimulation of respiratory center but later a metabolic acidosis becomes the dominant change.
- In liver failure.
- At high altitude in which the low oxygen content of the air stimulates respiration that causes excess loss of carbon dioxide and development of mild respiratory alkalosis.
3. Metabolic acidosis:
Etiology:
- Accumulation of lactic acid (vigorous exercise, shock from any cause, acute alcoholic intoxication).
- Accumulation of ketone bodies in uncontrolled diabetes mellitus.
- Renal diseases: in which the kidneys are unable to excrete hydrogen ions at a normal rate as in renal failure, and renal tubular acidosis.
- Diuretics such as carbonic anhydrase inhibitor as acetazolamide (Diamox) which is used frequently to cause diuresis also cause a mild degree of acidosis. This occurs because inhibition of the carbonic anhydrase of the proximal tubular epithelium prevents adequate reabsorption of bicarbonate ions from the lumen of the nephrons of the kidney in the form of CO2. Loss of these ions into the urine causes a decrease in bicarbonate ions in the extracellular fluid, thus leading to acidosis.
- Loss of intestinal contents: From fistulae or by intestinal aspiration or in severe diarrhea that causes an excessive loss of bicarbonate ions present in the gastrointestinal secretion in the stool.
- Vomiting, if the vomitus contains the secretion of the intestinal tract (which is rich in bicarbonate), rather than the stomach contents only.
- Ingestion of toxins (salicylates, methanol, ethylene glycol).
[4]. Metabolic alkalosis:
Etiology:
- Abnormal loss of HCl as in nasogastric suction or in the course of prolonged or severs vomiting. This type of alkalosis occurs in newborn children who have pyloric obstruction.
- Excessive ingestion of alkaline drugs such as sodium bicarbonate for the treatment of peptic ulcer.
- Diuretics in general cause increased flow of fluid along the tubules and this increase in the flow usually causes great excess amounts of sodium ions to flow into the distal and collecting tubules, leading also to a rapid reabsorption of sodium ions from these tubules. This rapid reabsorption is coupled with enhanced hydrogen ion secretion because of the Na+- H+ counter-transport mechanism in the luminal membranes of the tubular cells that links hydrogen secretion to sodium reabsorption, hence excessive loss of hydrogen ions from the body and resultant extracellular fluid alkalosis.
- Excess aldosterone secretion by the adrenal glands. Aldosterone promotes extensive reabsorption of sodium ions and simultaneous excretion of potassium from the distal segments of the tubular system, but coupled with this reabsorption of sodium is increased secretion of hydrogen ions which promotes alkalosis.
- Chloride deficiency itself also leads to alkalosis because it stimulates the renal tubular reabsorption of bicarbonate. This is especially liable to occur with the use of high potency diuretics that block chloride reabsorption.
- Potassium depletion also gives rise to extracellular alkalosis. This is because extracellular K depletion encourages the transfer of hydrogen ions from plasma and extracellular fluid into the cells (intracellular acidosis) as well as their excretion in urine (acidic urine).
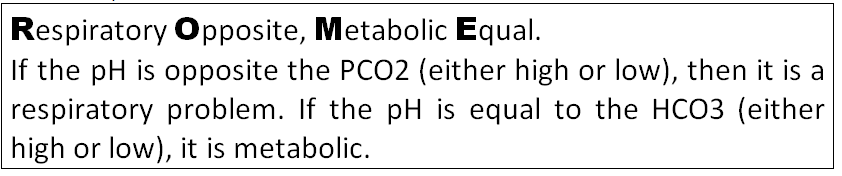
In summary for the acid-base abnormalities see figure. The easiest way to decide whether or not it is Respiratory
or Metabolic is by using the ROME technique.
Examples:
Match the acid-base status of the following blood samples to the acid-base disorder, (PCO2 values are in mm Hg and bicarbonate values in mmol/l). UC = Uncompensated, PC = partially compensated, FC = fully compensated.
- 1. pH 7.5, PaCO2 50, HCO3- 35
- 2. pH 7.6, PaCO2 18, HCO3- 20
- 3. pH 7.2, PaCO2 60, HCO3- 22
- 4. pH 7.4, PaCO2 58, HCO3- 33
- 5. pH 7.54, PaCO2 30, HCO3- 23
- 6. pH 7.4, PaCO2 25, HCO3- 16
- 7. pH 7.25, PaCO2 40, HCO3- 16
- 8. pH 7.55, PaCO2 52, HCO3- 40
- 9. pH 7.1, PaCO2 16, HCO3- 5
- 10. pH 7.25, PCO2 24, HCO3– 10
- 11. pH 7.52, PCO2 20, HCO3– 16
- 12. pH 7.69, PCO2 48, HCO3– 57
- 13. pH 7.50, PCO2 20, HCO3– 15
- 14. pH 7.28, PCO2 70, HCO3– 32
- 15. pH 7.12, PCO2 60, HCO3– 19
See the answers